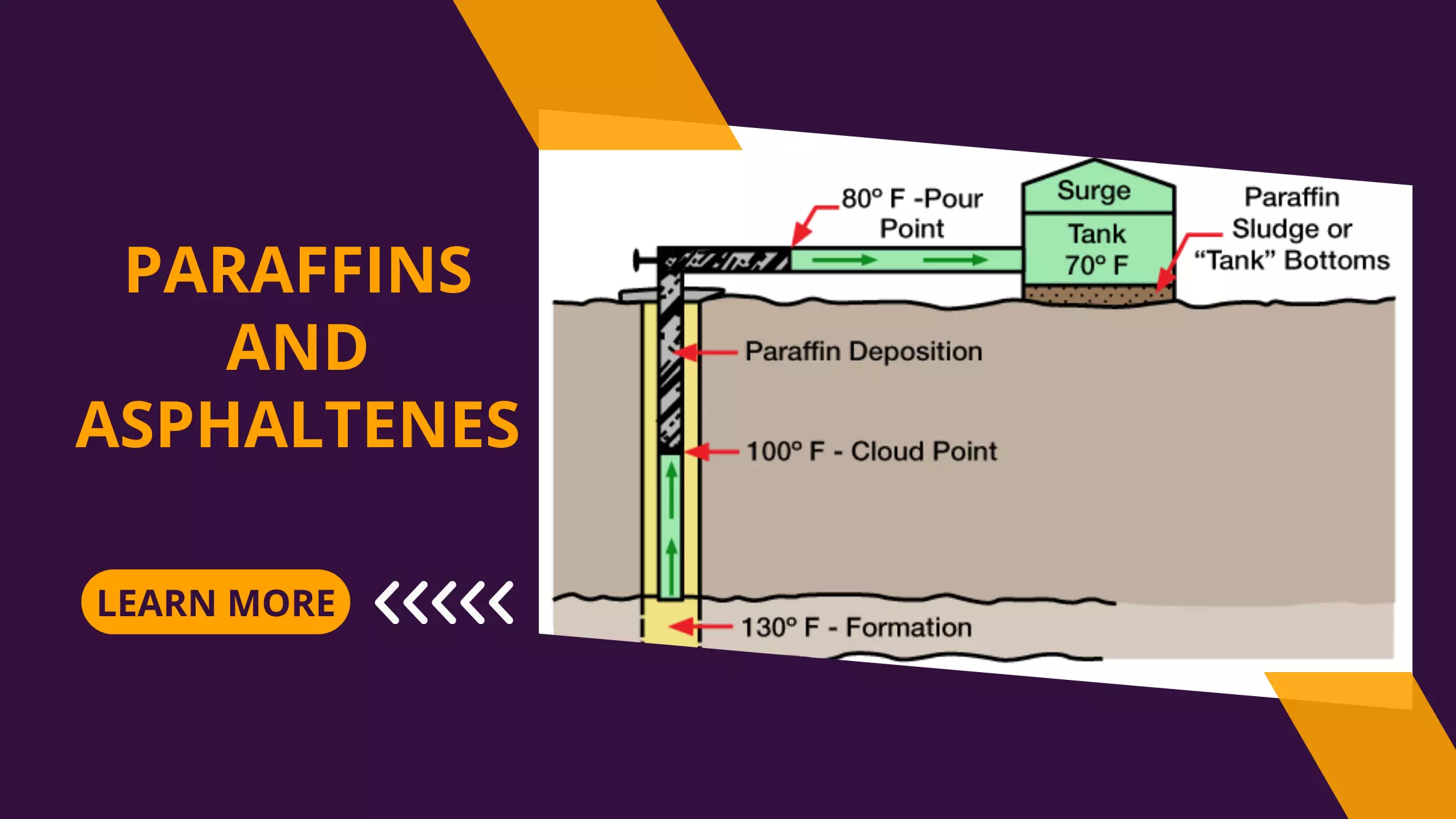
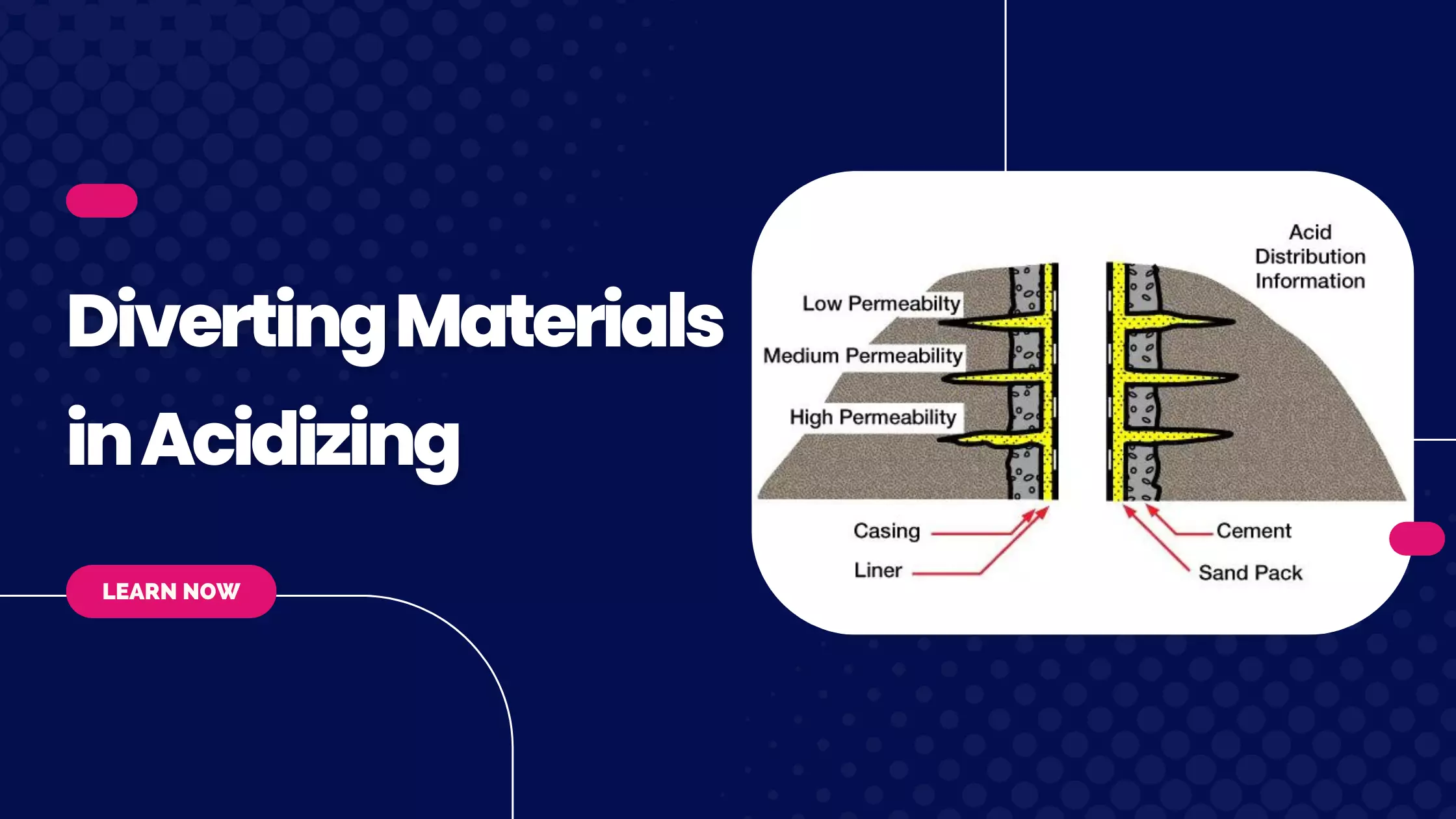
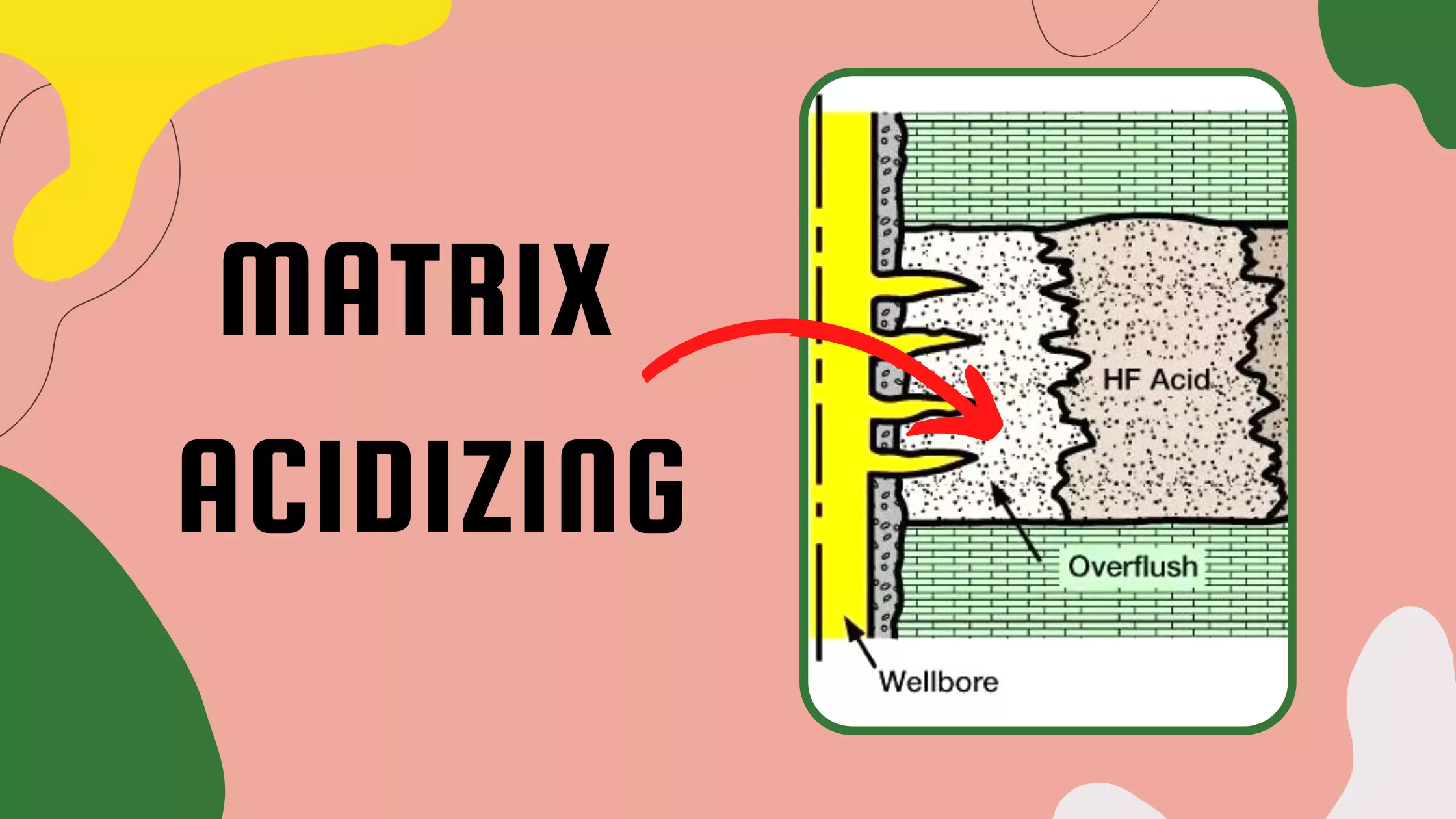
Formation Characteristics
Other important considerations in fracture acidizing are the following formation characteristics: rock composition, permeability, and temperature.
Rock Composition: The physical and chemical composition of the rock influences the reaction of acid; for example, dolomite is generally slower to react with HCl than limestone.
Carbonate formations are often mixtures of dolomite, limestone, and acid-insoluble materials. The more insoluble material a formation contains, the slower its reaction with acid. If the acid-soluble portion is lining fractures in an otherwise insoluble formation, excellent stimulation may be obtained even though total solubility is low.
Although carbonates seldom contain large quantities of clays, occasionally they can contain enough to deter the fracture acidizing treatment. If clays are present, potassium chloride can be added to the preflush and afterflush solutions. If iron compounds are present, iron sequestering agents added to the acid will inhibit the secondary precipitation of iron compounds when the acid spends.
Permeability: The matrix permeability dictates both the fluid properties and the amount of fracture conductivity needed to obtain the desired production rate. High permeability decreases fluid efficiency.
Temperature : Under normal conditions, acid reacts more quickly with carbonate materials as temperature increases. At extremely high temperatures, however, fluid viscosity decreases, which decreases fracture penetration significantly. Figure 1 contains data on the relationship between acid penetration distance and temperature.

 Petro Shine The Place for Oil and Gas Professionals.
Petro Shine The Place for Oil and Gas Professionals.