Specialty Acids
Specialty acids are designed to deal with specific formation conditions, such as deep clay damage, paraffin blockage in the reservoir, and situations requiring a retarded acid.
Powdered Acid
Powdered acids (sulfamic and chloroacetic) are used in applications where logistics are of primary concern – for example, in remote locations where bulk transport of liquids is impractical or very expensive. These crystaline powders are readily soluble in water.
Sulfamic acid (HSO3NH2) is a nonvolatile, nonhydroscopic, white crystalline or granular solid. Its reaction products with carbonates are water soluble, and it is highly ionized in water. Sulfamic acid is less corrosive than HCl, yet its strength is about the same. At 77° F a saturated solution of sulfamic contains about 18% hydrochloric acid equivalent.
Sulfamic acid reacts with limestone to form calcium sulfamate cvs, water and carbon dioxide
![]()
![]() (7)
(7)
One advantage of sulfamic acid is its solid physical state. Solid sulfamic acid can be transported and stored without special equipment or tanks, and mixed with water at or near the wellsite as needed. Sometimes it is cast into “acid sticks” for easy introduction into the wellbore.
The disadvantages of sulfamic acid include its decomposition at around 180° F, making it inappropriate for applications where formation temperatures are above 160° F. Moreover, HCl offers more dissolving power and comparable reactions at lower cost.
Chloroacetic acid is generally preferred to sulfamic acid when use of a powdered acid is appropriate, because it is stronger and more stable.
Retarded Acids
Retarded HF acid can penetrate deeper into a formation than conventional HF to remove siliceous solids. Retardation of HF achieves deeper penetration of unspent acid, can aid more complete formation damage removal, and further increase production.
A number of retarded HF acid systems are commercially available several of which are:
- SGMA (self-generating mud acid): The first retarded sandstone-acidizing system to be used extensively was developed by Shell Oil Company. It involves pumping ammonium fluoride and an organic ester, methyl formate, into the formation. (Methyl formate has a very low flash point and should be pumped with caution.)In time, ester hydrolysis produces formic acid. This acid reacts with ammonium fluoride to form HF, which then dissolves clays or any siliceous minerals it contacts.
- Claysol retarded HF(Halliburton): A retarded clay-dissolving system which utilizes ion-exchange properties of clay minerals to generate HF on clay in situ. Since HF is formed on clay surfaces, little sand is dissolved by this process. HF is created by sequentially injecting a volume of 3% ammonium fluoride followed by an equal volume of 5% HCl. This process dissolves clay in the formation as deep as a set of sequences can be pumped without completely mixing together.
- Clay acid(Dowell-Schlumberger): A retarded acidizing fluid using fluoboric acid (HBF4) for matrix acidizing of sandstone formations. HBF4 is usually applied as an afterflush to an HF treatment. Upon entering the formation, HBF4 slowly hydrolizes to generate HF. Deep acid penetration to remove fines is possible because of the slow generation of HF. Unlike clay-control agents, HBF4 also produces a chemical fusion of both fines and clay platelets, which provides fines/clay stabilization.
- RHF acid solution(Nowsco): A retarded HF system for treating sandstone formations suffering deep damage caused by migration and/or swelling of siliceous minerals. This single-stage treatment does not require sequencing or “shut-in” time for hydrolysis reactions. Addition of aluminum chloride(AlCl_3) to an HF acid solution forms aluminum fluoride complexes, similar to those formed in spent regular mud acid, which retards the reaction rate of HF with siliceous materials.
Retarded Hydrochloric Acid
The HCl reaction rate on carbonate formations can be retarded by gelled acid, oil-wetting formation solids, or emulsifying acid with hydrocarbon.
Gelled Acid Systems: Gelled acids are used to retard acid reaction rate during fracture acidizing. Retardation occurs because increased fluid viscosity reduces the rate of acid contact with the fracture face.
Other related advantages include:
- reduced leak-off rate
- deeper penetration
- better cleanup of fines or solids transport
Viscosifying agents normally associated with gel led acid systems consist of natural polymers, synthetic polymers, cross-linking agents, and surfactant gelling chemicals.
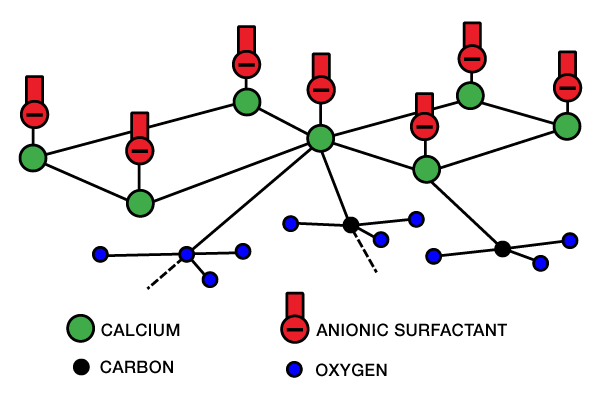
Chemically Retarded Acids: When it is desirable to extend the spending time of an acid system, a chemical retarding agent may be used. Most chemical retarders are anionic surfactants (such as sulfonates or sulfates). These oil-wetting surfactants adsorb onto a carbonate to create a physical barrier to acid contact with the rock surface. The adsorption mechanism on a calcium crystal may look like the illustration in Figure 1 (absorption mechanism).
Emulsified Acids: Emulsified acids, obtained by emulsifying acid and a hydrocarbon, are effective over a wide range of bottomhole temperatures. The system may be either oil or acid external, but the most common form is an oil-external emulsion.
- Oil-external emulsion is composed of hydrocarbon-base fluid (refined or crude) as the continuous phase and HCl as the internal phase. This acid system retards the reaction of acid with the formation by decreasing the amount of acid in contact with the rock. As the acid spends, the emulsifier reacts with the resulting CaCl2 solution, releasing its emulsifying properties and therefore causing the emulsion to break.
- Acid-external emulsion is composed of acid as the external phase, where selection of acid depends on the well conditions involved. The acid phase may account for 80 to 90% of its total volume. Ordinarily, aromatics, such as toluene or xylene, are used as the hydrocarbon or internal phase. This acid system is used primarily to remove hydrocarbon materials like paraffin, congealed oil, and other deposits so acid can contact acid-soluble materials.
Foamed Acid
Foamed acid has widespread application for effective fracture acidizing in carbonate reservoirs. Both oil and gas wells have responded successfully to foamed acid stimulation treatments. Foamed acid is particularly beneficial in low-pressure, low-permeability, liquid-sensitive formations because of the following advantages:
- High Viscosity: While often difficult to achieve with conventional gelling agents in acid, high apparent viscosities are easily developed by foaming the acid. Higher viscosities may result in wider fractures, better fluid-loss control, and better solids-carrying capabilities.
- Reduced Fluid Loss: The high apparent viscosity of foamed acid results in reduced fluid loss, which allows deeper acid penetration. In low-permeability reservoirs, foam bubbles may be sufficient to decrease leakoff into the formation matrix.
- Improved Solids Transport: In conventional acid treatments, large amounts of insoluble fines can be left in the formation because of low-viscosity spent acid. These solids can reduce fracture conductivity. Foamed acid and high viscosity suspend and remove more fines from the well during cleanup.
- Better Cleanup: Foamed acid offers built-in gas assist. This means faster cleanup. It may even eliminate the need to swab the well after acidizing.
- Less Damage: Foamed acid has a low liquid content. Normally, the volumetric gas content(referred to as foam quality) is 60 to 80 quality (i.e., 60% to 80% gas and 40% to 20% acid).
Experience with foamed acid has shown that increasing acid viscosity with conventional gelling agents before foaming can help increase foam stability. The longer foamed acid, live or spent, is allowed to remain static, the easier it is for acid to drain from the foam bubbles. This foam destabilization (liquid/gas separation) allows suspended fines to settle out.
Nitrogen is the most common gas used to make foamed acid. Foam quality is generally between 60% and 80%, although qualities as high as 95% have been used. The acid phase of the foam may contain 0.5% to 1.0% surfactant and 0.2% to 2.0% corrosion inhibitor.
Turflo/Hydrofluoric Acid
As stated earlier, HCl–HF mixtures react with both siliceous and carbonate materials. In most sandstone formations carbonate content is small, and an HCl preflush removes all carbonate material. HCl may not, however, do the job effectively in formations with high limestone or dolomite content. These carbonates can reduce the effectiveness of a HCl–HF treatment by increasing the chance of secondary reactions and by limiting the amount of clays removed.
Turflo/hydrofluoric acid, a phosphoric acid-HF combination, is specifically designed for formations with high carbonate content. Tests with this system have shown its reaction rate is much slower than that of normal 12% HCl-3% HF with limestone and nearly as fast with silicas.
Since Turflo/HF is slightly slower reacting than HF acid, deeper penetration of HF is possible. Turflo/HF is more expensive than conventional HF, but its limited reaction rate with carbonates allows for improved formation damage removal in sandstone formations with high carbonate content.. However, caution should be exercised because precipitation problems may subsequently cause severe permeability damage.
 Petro Shine The Place for Oil and Gas Professionals.
Petro Shine The Place for Oil and Gas Professionals.