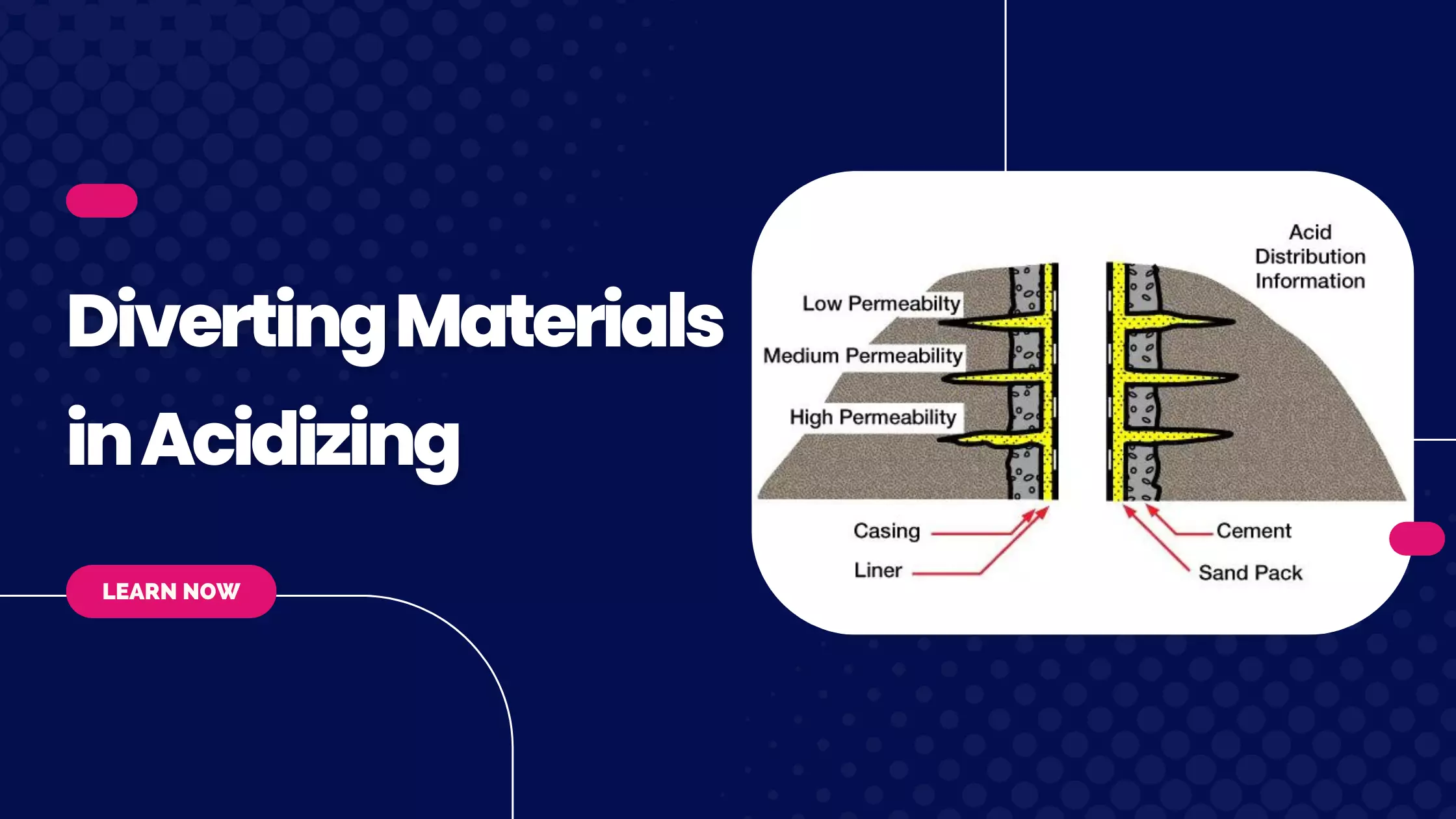
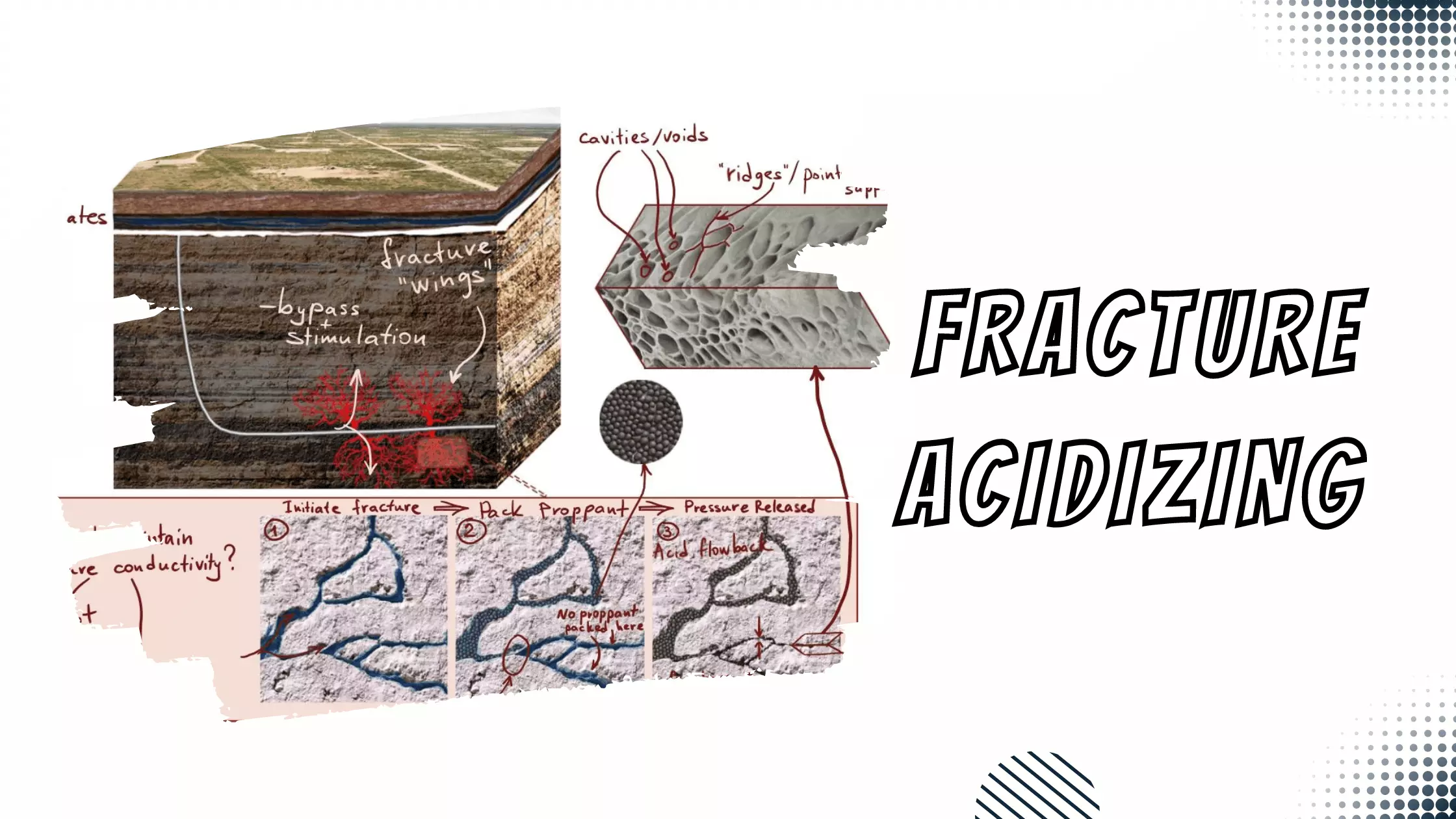
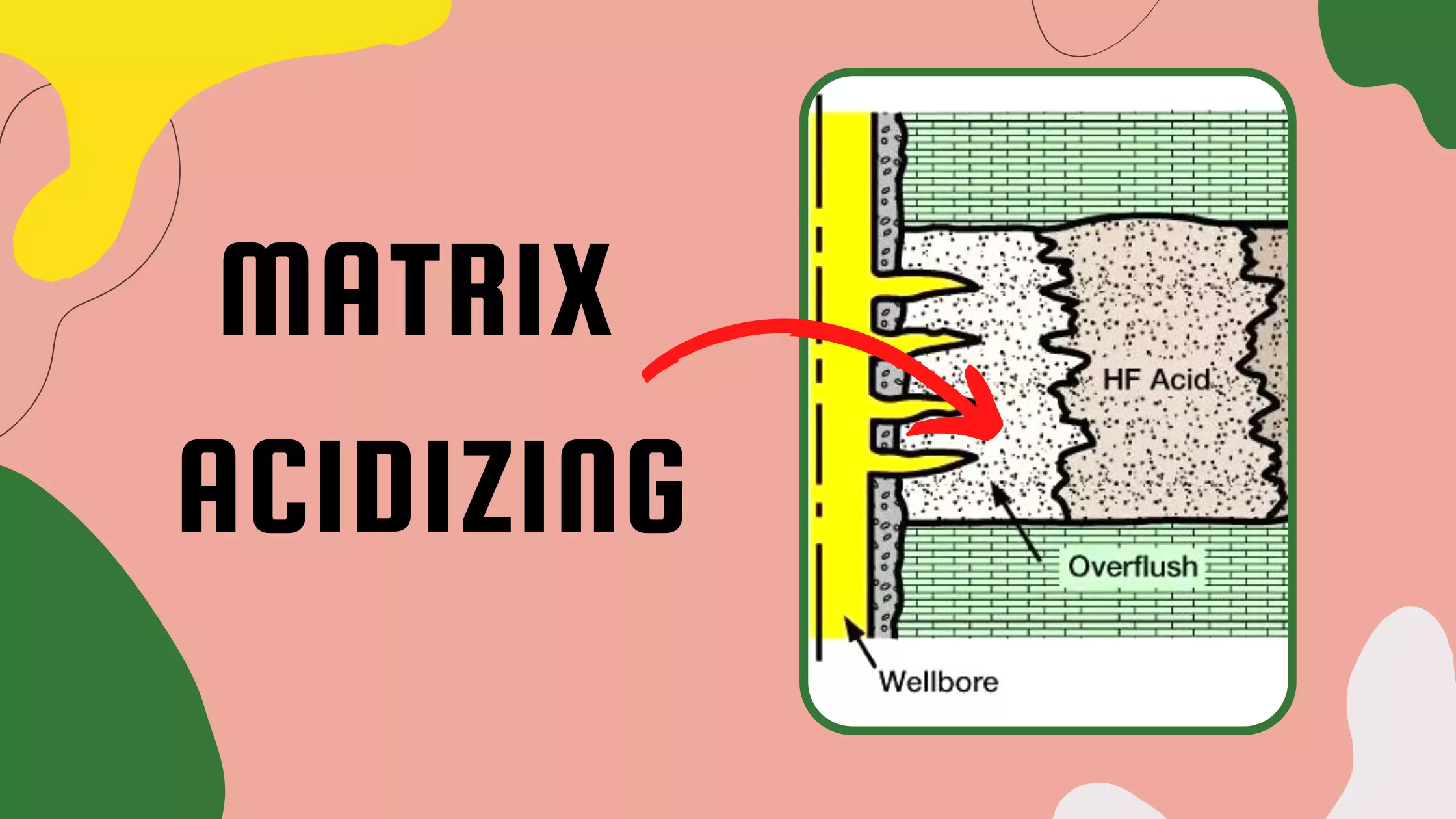
Basic Properties
Paraffins are organic deposits caused by changing wellbore conditions that upset the chemical equilibrium so that various materials in the crude oil precipitate out of solution. These materials separate and deposit a waxy material called paraffin (in some cases asphaltene). Figure 1 illustrates the development of paraffin in a flowing well.
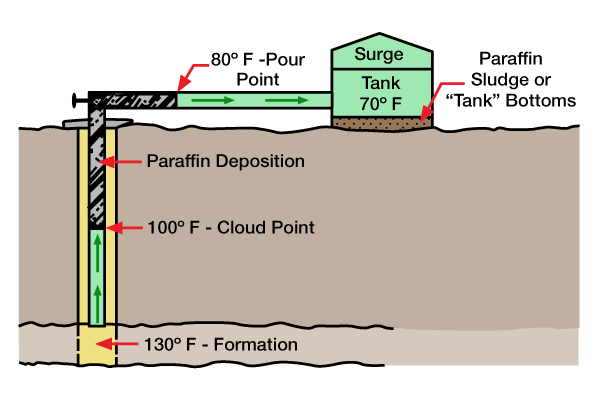
Paraffin, usually deposited in the wellbore, extends to the top of the well and into flowlines from a depth where the formation temperature is approximately the same as that at which the oil became saturated with the separated wax.
Asphaltene deposits, usually hard and brittle, occur at the bottom of the wellbore adjacent to the producing formation wall.
The buildup of paraffin or asphaltene can completely plug up the oil flow in a producing well if the problem is ignored or left untreated.
Properties
Paraffins are either normal, branched, or cyclic alkanes with the chemical formula CnH2n+2. Paraffin deposition is difficult to inhibit because these hydrocarbons are generally very inert and therefore resistant to attack by acids, bases, and oxidizing agents.
Crude paraffin deposits are made up of individual alkanes ranging from a carbon chain length of 20 with a melting point of 98° F to a chain length of 60 with a melting point of about 215° F. Crude wax or paraffin deposits consist of very small crystals that tend to agglomerate and form granular wax particles about the size of ordinary table salt grains. Deposited paraffin may also contain gums, resins, asphaltic material, crude oil, sand and silt, corrosion products, scale, and often water.
The primary cause of wax separating from crude oil is loss of solubility, often resulting from changing environmental conditions that disturb solution equilibrium. Factors affecting this equilibrium include temperature and pressure changes, evaporation, and loss of dissolved gases. Those paraffins having the highest melting point and molecular weight are the first to separate from solution. The quantity of lower melting point waxes with smaller molecular weights to separate from solution depends on how much the oil’s equilibrium changes prior to deposition – that is, the solubility of different paraffin waxes in specific crude oil at a given temperature decreases with an increase in melting point and molecular weight.
Asphaltenes are black carbonaceous petroleum components characterized by relatively high molecular weights and insolubility in light paraffinic hydrocarbon solvents, such as pentane and petroleum ether. The asphaltene fraction of petroleum is made up of polycyclic, condensed aromatic rings with few side chains. These compounds are considered polar materials due to the presence of sulfur, nitrogen, oxygen, and complex metals.
Asphaltenes occur in many crude oils in the form of colloidally suspended solid particles. Asphaltene precipitation takes place when crude oil loses its ability to disperse the particles. Asphaltene stability is dependent on crude oil composition and the nature of reservoir rock surface. The balance that holds them in a stable suspension is dependent on the same conditions that cause paraffin to separate from crude.
Pour point and cloud point measurements refer to crude oil’s ability to hold paraffin in solution. Cloud point is the temperature at which paraffin begins to come out of solution. This point is visible in clear solutions as a slight cloudiness. Since the cloud point of a dark crude oil is not visible, instrumental methods may be used to obtain crude oil cloud points.
If crude oil is slowly cooled below the cloud point without agitation, small wax crystals gradually form an interlocking network which inhibits flow. The pour point is reached when oil ceases to flow.
 Petro Shine The Place for Oil and Gas Professionals.
Petro Shine The Place for Oil and Gas Professionals.