Paraffins Removal and Control Techniques
There are basically three methods for removing and/or controlling paraffin deposition: mechanical, thermal, and chemical.
Mechanical Methods: Several mechanical techniques remove paraffin deposition in tubing, flowlines, and pipelines. These include
- rod scrapers
- wireline scrapers
- flowline scrapers
- balls, pigs, and/or soluble plugs
- free-floating pistons
- hollow rods for circulating hot fluids
The major advantage of using mechanical methods is that positive cleaning is assured. There are some disadvantages, however. Application is rather limited because of the time, expense, personnel, and special equipment involved. If tools are lost in the hole, a fishing job may be necessary.
One preventive mechanical method is the use of smooth-surface pipe. The internal surface of pipe can be made smooth by plastic coating or removal of mill scale. Smooth pipe surfaces do not prevent paraffin deposition, but in most cases they slow the process.
There are three widely used plastic coatings for pipe: phenol-formaldehyde, epoxy phenolic, and polyurethane.
- Phenol-formaldehyde has been in use longest, and is the most widely used coating in oilfield tubulars. It is a highly crosslinked polymer, with excellent resistance to temperature, chemicals, and infusion by small molecules (H2O, CH4, H2S). Its surface is extremely smooth and has a high gloss. It is a brittle plastic which becomes extremely rough when abraded by sand.
- Epoxy phenolic is the second most widely used material in oilfield tubulars. Less resistant to temperature, chemicals, and infusion than phenol-formaldehyde, its surface is almost as smooth, but it is less brittle and does not deform as badly when abraded by sand.
- Polyurethane is a relative newcomer, and the least crosslinked of the three. It has lower temperature, chemical, and infusion resistance than the other two, and its surface is not quite as smooth as phenol-formaldehyde. Polyurethanes are quite flexible, and although (like the other two materials) its film thickness is reduced by sand abrasion, the plastic is only slightly deformed and maintains most of its smoothness.
Plastic-coated pipe either works extremely well or is a dismal failure. Numerous examples of both cases can be found in oil-field operations. Given the descriptions above, it is apparent why particular plastics may perform well or not, depending on their application and the experience of the particular oil operator. In areas where the oil stream contains no sand or other abrasive solids, plastics effectively prevent paraffin buildup. When brittle plastics (such as phenol-formaldehyde) are used in wells containing abrasive materials, the plastic deforms badly, leaving a rough, abraded surface which only invites severe paraffin deposits.
There are three important factors regarding the use of plastic coatings in the prevention/reduction of paraffin deposition:
- Paraffinic plastics such as polytetrafluoroethylene, polyethylene, and polypropylene, no matter how smooth, do not reduce paraffin buildup. In fact, because they are themselves, they cause paraffin buildup by either hydrogen bonding or a phenomenon closely resembling cocrystallization.
- Smooth, nonparaffinic plastics reduce paraffin deposits only as long as they remain smooth. Wirelines, fishing tools, piano wire equipment, and other workover or measuring devices destroy the effectiveness of the plastic coating by damaging the smooth surface.
- All metal surfaces(nipples, valves, etc.) must be coated with plastic. One uncoated nipple can effectively choke the well.
Thermal Methods: All thermal techniques use heat to remove the paraffin. Methods in current use include
- bottomhole heaters
- hot oil, a common method which melts the waxy accumulation; lease crude oil is run through heat exchangers and pumped into the well at temperatures greater than 300 °F
- steam, which melts the paraffin so that it can be flowed with the oil from the well
- heat-liberating chemicals(such as magnesium bars) followed by HCl; these produce chemical reactions which release heat, thereby melting the paraffin
These approaches ensure paraffin removal. In order to prevent further deposition, the working temperature must be maintained above the melting point of the wax.
Thermal techniques have some disadvantages. Bottomhole heaters require special equipment and consume additional power. Hot fluids pose a danger to personnel, usually require the use of pump trucks, and sometimes dissolve the paraffin only to redeposit it deeper than before.
Chemical Methods: Chemical methods for paraffin control are separated into two distinct categories: solvents and inhibitors.
Solvents: The best solvents for paraffin removal are too toxic, hazardous to handle, or damaging to refinery catalysts.
Two such chemicals are carbon disulfide (CS2) and carbon tetrachloride (CCl4).
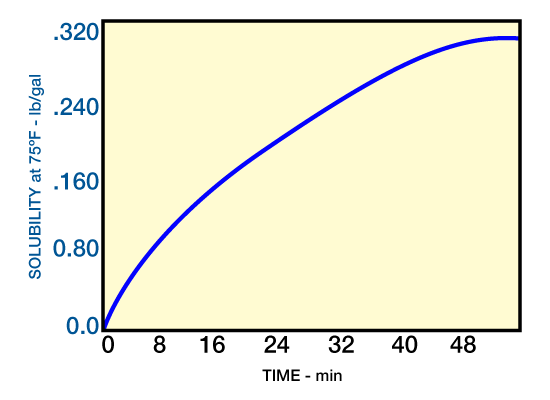
Aromatic hydrocarbons, such as xylene and toluene, are acceptable solvents and dissolve both wax and asphaltenes; they are suitable for crude paraffin deposits. Solubility of paraffin in xylene is shown in Figure 1.
Inhibitors: A large number of paraffin inhibitors are also available. Explanations of their effectiveness vary widely. Some are said to be dispersants; others, wax crystal modifiers; and still others claim to alter the surface conditions of equipment. Most paraffin inhibitors also contain paraffin solvent as the carrier.
- Surfactants: Of the vast number of paraffin chemicals available, wetting agents and dispersants constitute the highest percentage. Wetting agents are designed to work much like plastic pipe: the chemical reacts with produced water or by itself and forms an aqueous film on the pipe surface. This surface film retards paraffin deposition by covering the sites where paraffin can adhere. However, it is very difficult to get the pipe surfaces water-wet and keep them that way. The theory behind the dispersant approach is that certain chemicals tend to cause the crude paraffin molecules to repel each other as well as the metal surface.
- Both wetting agents and dispersants have seen some success. The most serious drawback to their use is that no supplier can be absolutely certain of their effectiveness. The operator has to decide whether to continue a particular paraffin removal program or to run the trial and error method with all available chemicals and hope to find one that is satisfactory.
- Crystal Modifiers: Generally, crystal modifiers have many of the same drawbacks as surfactants. Their effectiveness appears to be somewhat unpredictable. With the proper placement technique, however, these chemicals seem to work fairly well and to show the most promise among chemical inhibitors. When viewed under a microscope, they appear to modify the wax crystals precipitating from a wax solution (Figure 2, paraffin crystal clustering and Figure 3, effect of inhibitor on paraffin crystals).
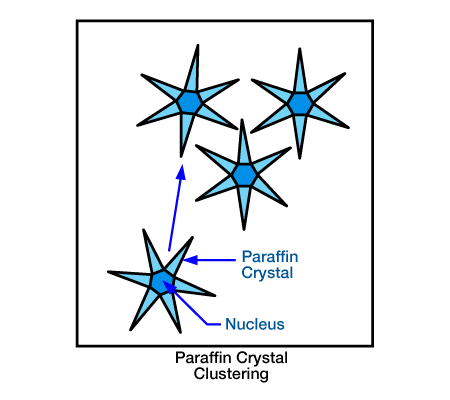
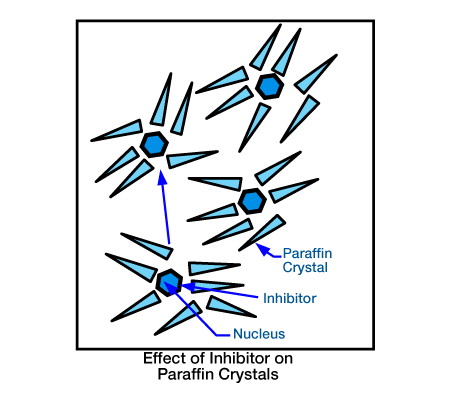
- Crystal modification occurs for one of two reasons: (1) the chemical comes out of solution at a temperature slightly higher than that required for paraffin precipitation, causing nucleation, or (2) the modifier comes out of solution and co-crystallizes with the paraffin crystals. In either case, the normal crystal habit of paraffin is deformed sufficiently to inhibit further growth. This causes the crude oil to become saturated with much smaller paraffin crystals than normal with less tendency to adhere to pipe surfaces. Although they do not completely cure the paraffin problem, crystal modifiers offer the best chemical approach for reducing its severity.
 Petro Shine The Place for Oil and Gas Professionals.
Petro Shine The Place for Oil and Gas Professionals.