Maturation processes refer to the natural physical and chemical changes that occur in organic matter over time as it is buried deeper and subjected to higher temperatures and pressures. These processes are important in petroleum geology because they can lead to the formation of hydrocarbons (oil and gas) from organic-rich source rocks.
Kerogen Maturation
Let’s consider the chemical variability of kerogen types and the changes produced as petroleum is generated. To do this, we use Van Krevelen plots, which graph the atomic hydrogen to carbon ratio ![]() versus the oxygen to carbon ratio
versus the oxygen to carbon ratio ![]() (Figure 1,
(Figure 1, ![]() and
and ![]() ratios of kerogen Types 1-IV and their stages in the evolution of petroleum.
ratios of kerogen Types 1-IV and their stages in the evolution of petroleum.

Arrows show evolution paths at increasing maturity. O: formation of oxygenated products [CO2, H2O, NSO molecules]; P: petroleum formation; G: gas formation.). The ![]() ratio helps define the origin of kerogen. Most of the oxygen is lost during diagenesis in the form of carbon dioxide and water. The highest organic
ratio helps define the origin of kerogen. Most of the oxygen is lost during diagenesis in the form of carbon dioxide and water. The highest organic ![]() ratio of 4 is found in methane gas. The
ratio of 4 is found in methane gas. The ![]() ratio decreases rapidly as hydrogen-rock molecules crack off as oil or gas.
ratio decreases rapidly as hydrogen-rock molecules crack off as oil or gas.
Type I algal kerogens enter diagenesis with the highest atomic ![]() ratios of about 1.65, while Types II, III and IV enter diagenesis with progressively lower
ratios of about 1.65, while Types II, III and IV enter diagenesis with progressively lower ![]() ratios. As any one of these kerogen types is heated, it may reach the second stage in the formation of petroleum, catagenesis (Figure 2).
ratios. As any one of these kerogen types is heated, it may reach the second stage in the formation of petroleum, catagenesis (Figure 2).
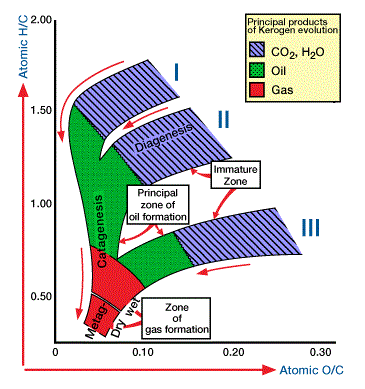
Because oil and gas molecules have very high ![]() ratios, petroleum generation causes the
ratios, petroleum generation causes the ![]() ratio of the residual kerogen to decrease. Generation of oil and gas from kerogen ceases during the final stage of petroleum generation, metagenesis, but large quantities of methane gas can be generated by thermal alteration of previously generated crude. Residual kerogen produced during this stage approaches pure carbon in the form of graphite.
ratio of the residual kerogen to decrease. Generation of oil and gas from kerogen ceases during the final stage of petroleum generation, metagenesis, but large quantities of methane gas can be generated by thermal alteration of previously generated crude. Residual kerogen produced during this stage approaches pure carbon in the form of graphite.
Although both Type I and Type II kerogen are oil-prone, Type I kerogens generate more hydrocarbons than Type II kerogens because ![]() ratios are initially higher for Type I kerogens. Likewise, Type II kerogens generate larger quantities of hydrocarbons than Type III kerogens, and Type IV kerogens are essentially barren.
ratios are initially higher for Type I kerogens. Likewise, Type II kerogens generate larger quantities of hydrocarbons than Type III kerogens, and Type IV kerogens are essentially barren.
 Petro Shine The Place for Oil and Gas Professionals.
Petro Shine The Place for Oil and Gas Professionals.



