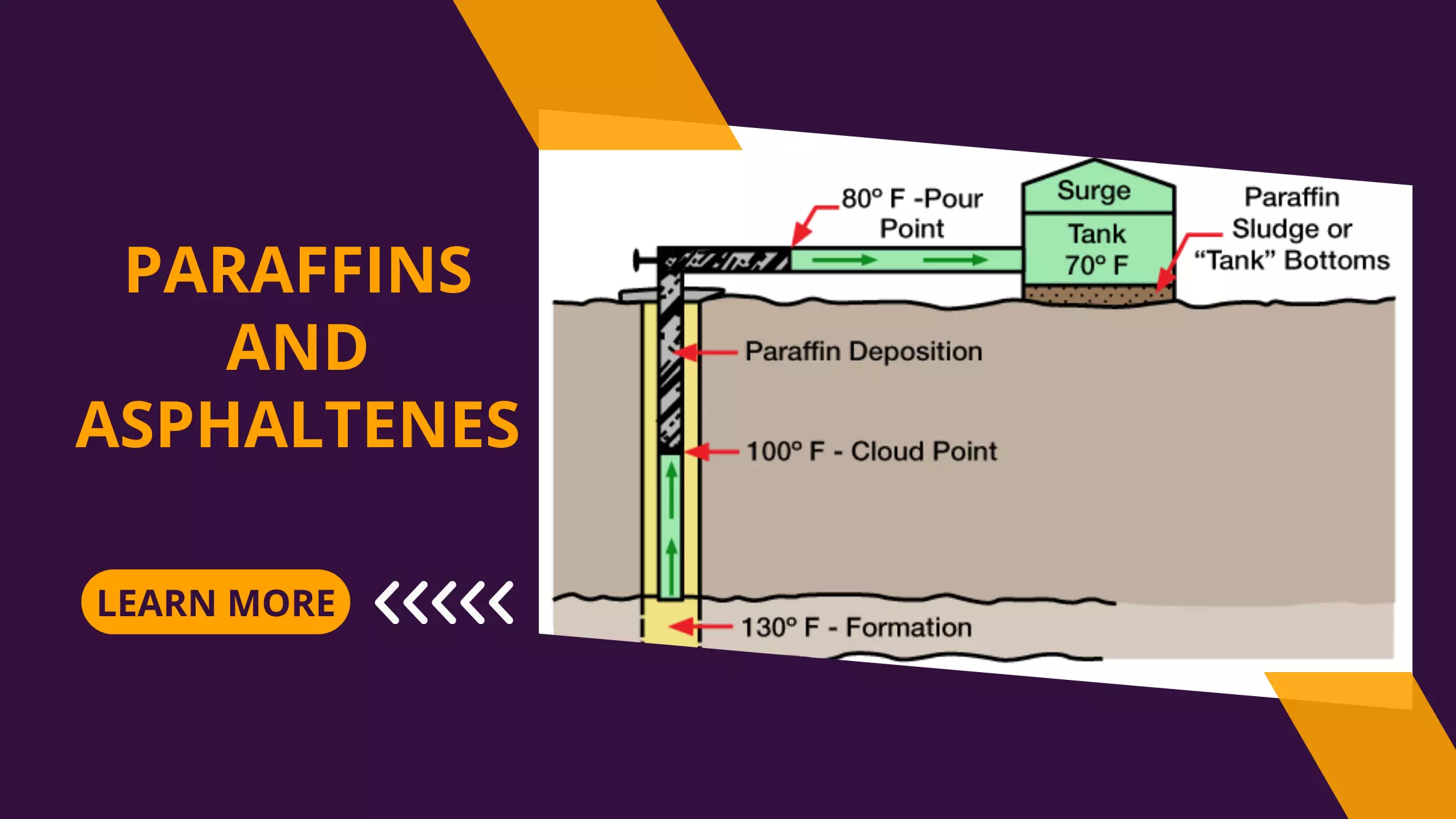
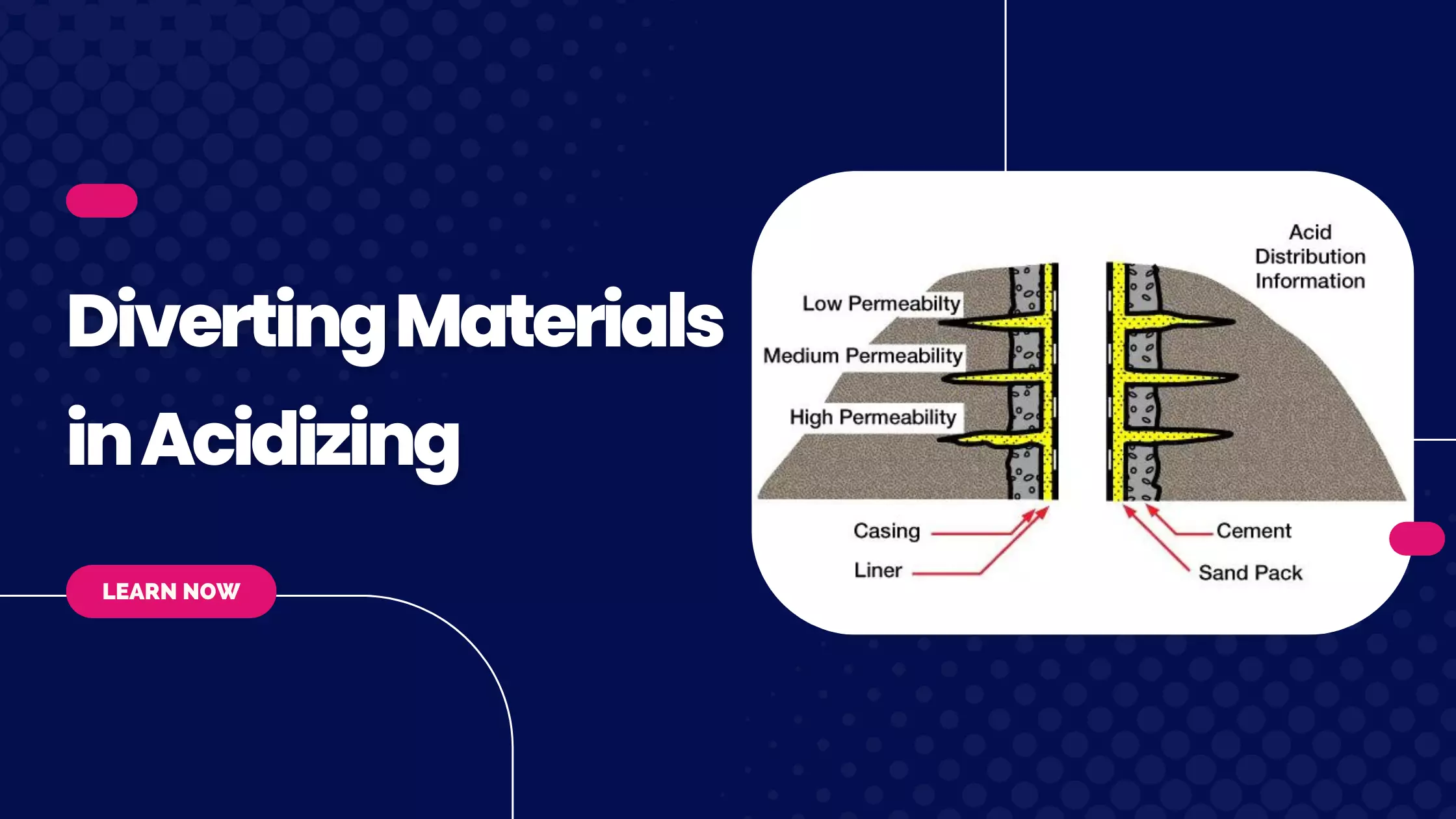
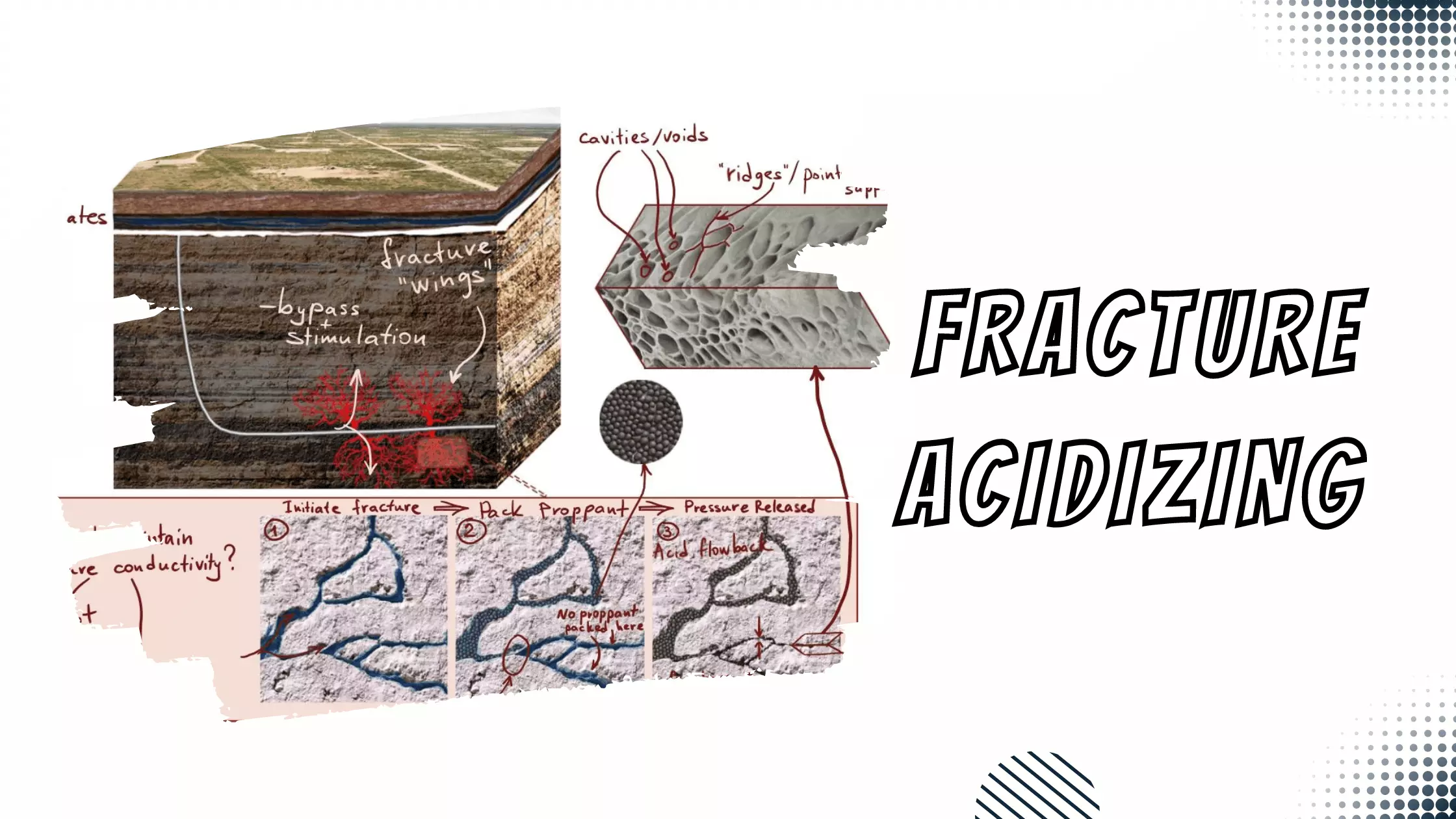
Clay Stabilizers
Many clay-stabilizing materials have been developed in recent years to combat formation damage caused by clay swelling and migration. These generally are used in conjunction with minimum-salinity fluids such as 2% KCl, 4% NaCl, and 3% NH4Cl.
Types of Clay
Some clays, like smectite-mixed layer, swell when they contact fresh water or water with low salt concentrations. There are several ways in which water can enter and damage a productive zone. Some of these are:
- water filtrate from drilling fluid;
- loss of water from completion or workover fluids;
- encroachment into the reservoir by water coning;
- any type of water-base treating fluid.
Productivity also decreases when the produced fluid carries the clay fines (colloidal-size clay particles) in suspension to the wellbore. When any type of pore restriction is encountered, the fines bridge off in the pore, thereby plugging the pore and restricting the flow. Radial flow volume of fines increases while the number of pore channels decreases. Some clays with migrating tendencies are illite, kaolinite, and chlorite.
Smectite-Mixed Layer: Smectite-mixed layer, a three-layer clay, is composed of two silica tetrahedral sheets with a central octahedral sheet (Figure 1, smectite -mixed layer).

In the stacking of their mineral sheets, the proximity of two oxygen layers makes for a weak chemical bond that allows water and other polar organic minerals to enter between unit layers and cause the clay to swell. Figure 2 (three layer clay) illustrates the bonding of smectite-mixed layers. The cation exchange sites attract such positive ions as sodium, potassium, calcium, or ammonium.
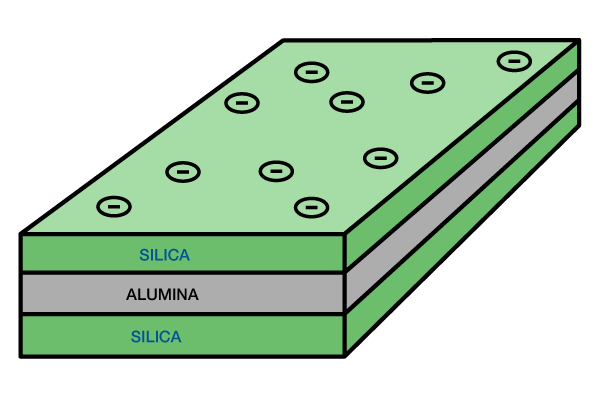
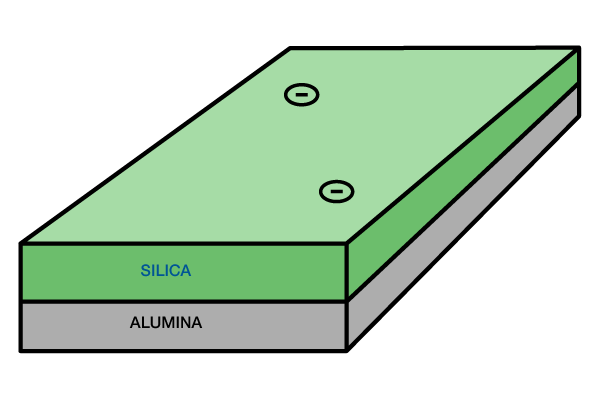
Illite: Illite is a clay composed of two silica tetrahedral sheets with a central alumina octahedral sheet (Figure 3).
Its structure is similar to smectite, except that potassium and oxygen bind the sheets tightly. Hence, this clay does not expand in the presence of water. However, the potassium ions are very susceptible to the leaching action of slightly acidic water. In some cases, the potassium may be removed and replaced by another cation. These altered minerals, called degraded illites, then allow entry of water and will swell as a result.
Kaolinite: Kaolinite is composed of one silica tetrahedral sheet and one octahedral sheet (Figure 4). Their tight hydrogen bonding is not susceptible to water entry. Although kaolinite swells very little on contact with fresh water, it is an important component of migrating fines.

Figure 5 (two layer clay) schematically illustrates a two-layer clay.
Kaolinite is the principal member of this family of clays. There are few cation exchange sites: 10% of the number for smectite-mixed layer – too few to cause significant swelling.
The degree to which a clay will swell depends on the ions the clay has adsorbed, the degree of degradation of the clay, and the amount of substitution in the clay lattice. The amount of swelling of several salts of smectite is shown for various concentrations in Table 1 (below). These data show that certain ions, especially sodium, allow swelling of clays, resulting in formation damage. In Figure 6 (salinity effects on clays), solutions of sodium chloride (NaCl) were flowed through columns of quartz sand containing 4% by weight of various clays.

These curves show that the higher salinity solutions of NaCl did less damage to the permeability of the columns than the lower salinity and fresh water systems.
| Salt Concentration | Sodium Smectite | Potassium Smectite* | Calcium Smectite |
|---|---|---|---|
| Distilled Water | 33 | 27 | 4 |
| 0.4% Chloride Solution | 14 | 2 | 4 |
| 2.0% Chloride Solution | 3 | 2 | 4 |
| 8.5% Chloride Solution | 2 | 2 | 4 |
| 10.0% Chloride Solution | 2 | 2 | 3 to 4 |
*Ammonium chloride will perform essentially the same as potassium chloride.
The data in Table 1 and Figure 6 show good agreement. Both indicate that low concentrations of salt swell the smectite more severely than do higher salt concentrations. Figure 6 also indicates that the sensitivity of the clays to water swelling is
kaolinite < illite < smectite
with kaolinite showing the least damage and smectite the most.
Clay damage may be caused by both swelling and migration. When these conditions occur in a formation, the damage must be removed to improve production. This is commonly accomplished by acidizing the formation with HCl-HF mixtures.
Clay Control
Formation damage due to clays must be prevented rather than cured. When clay particles in sandstone reservoirs are rearranged or disturbed in any manner, it is usually impossible to restore the original permeability.
X-ray diffraction analysis can easily determine the type and amount of clay in a particular sandstone. These tests indicate which formations warrant a particular minimum-salinity fluid, clay stabilizer, or special completion technique to avoid formation damage.
The position of clays in the rock can be quickly determined by the use of dyes that exhibit characteristic colors when absorbed by different types of clays. Where detailed studies are warranted, the scanning electron microscope (SEM) permits direct observation of certain actions on clays.
In a virgin formation, the swelling of a clay particle is in equilibrium with the type and concentration of salts in the connate water. Thus, filtered formation water used in workover operations should not disturb this balance. If clean formation water is not available, it is necessary to synthesize an economical substitute.
Cat ionic polymers have been developed that can plate out of fresh water, brine, or acid to stabilize clays and minimize damage. These cationic materials do not oil-wet because the exposed polymer prefers water over oil. The cationic ends of the clay stabilizers bind to the clay minerals in the same manner as the sodium Na+, potassium K+, or calcium Ca++ ions bind. This leaves the water-soluble end of the molecule to form a film over the clay minerals to prevent their swelling and migrating. The principal cationic polymers currently available for use in sandstone acidizing include polyquaternary amines (PQA) and polyamines (PA).
Polyquaternary Amines (PQA): PQA is a cationic organic polymer used as a clay-stabilizing additive in sandstone acidizing applications and other treatments. It is a clay-stabilizing chemical that can be universally placed out of aqueous solutions and strong acids.
Since PQA does not require a shut-in period, it allows faster well cleanup and prevents damage resulting from swelling and/or migration of clay particles and other silicate fines. The chemical action between PQA and clays results in longer stabilized production before migrating and/or swelling silicates begin to redamage the formation.
Figure 7 illustrates relative permeability in a Bandera sandstone core without any clay stabilization treatment.

Notice that after the core was calibrated with 2% KCl and then exposed to fresh water, permeability decreased about 85%.
Figure 8 demonstrates relative permeability in a Bandera sandstone core after a clay-stabilization treatment with PQA (polyquaternary amine).
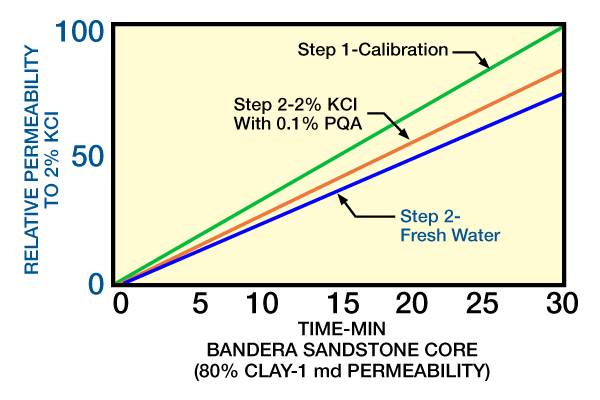
Following 2% KCl calibration and clay-stabilization treatment, fresh water had only minimal effect on the permeability, decreasing it slightly.
Although the PQA concentration needed depends on permeability, the usual maximum is 3% by volume.
All fluids containing PQA should be overflushed with a sufficient volume of fluid to ensure that they are displaced to a distance of at least two feet from the wellbore. This results in more fines stabilizing for a given PQA concentration.
Polyamines: These are cat ionic polyelectrolytes which stabilize water-sensitive clays against the swelling and migration problems that might occur on contact with aqueous fluids. PA can be used in aqueous preflush and overflush fluids and acids.
 Petro Shine The Place for Oil and Gas Professionals.
Petro Shine The Place for Oil and Gas Professionals.