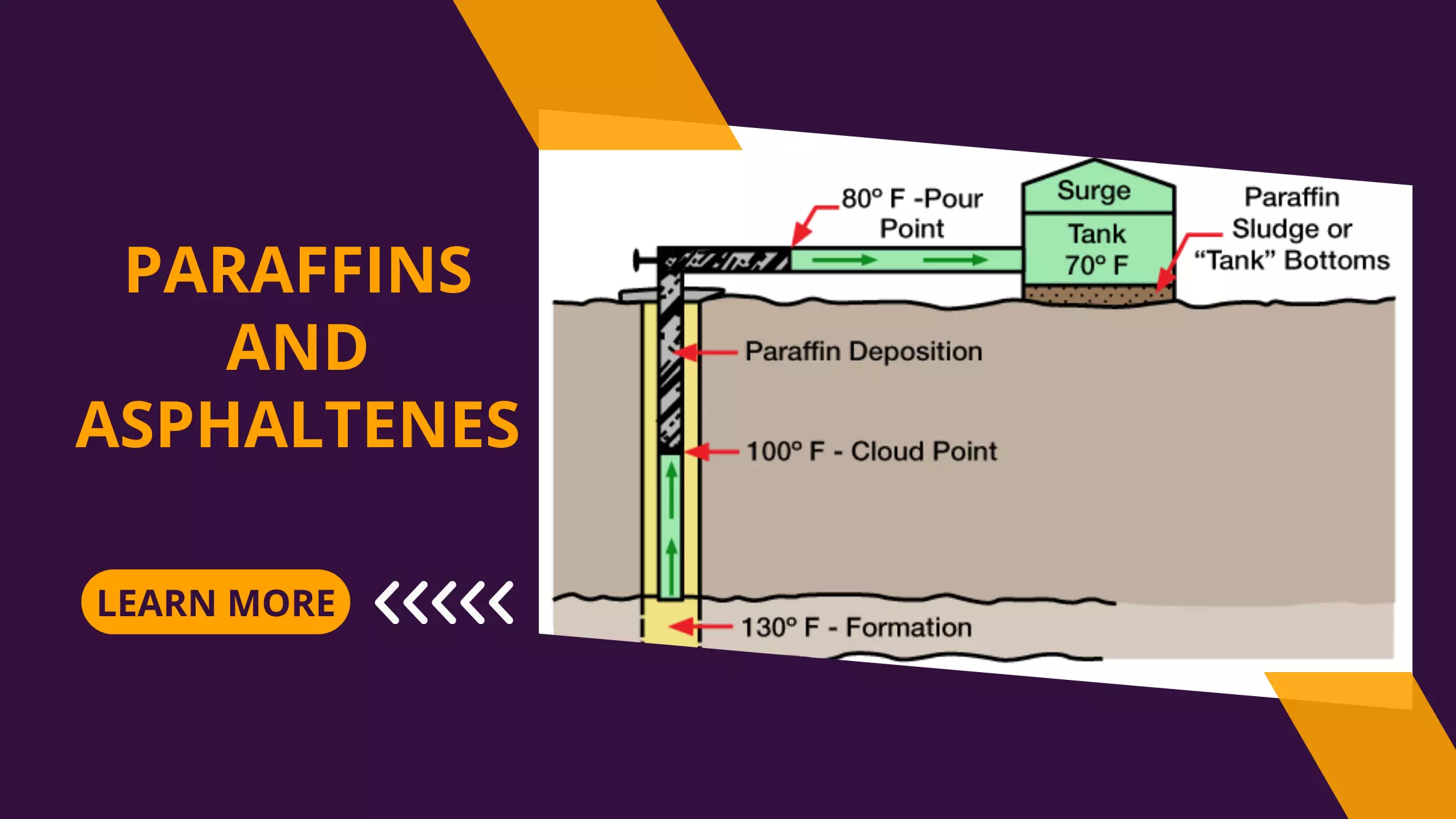
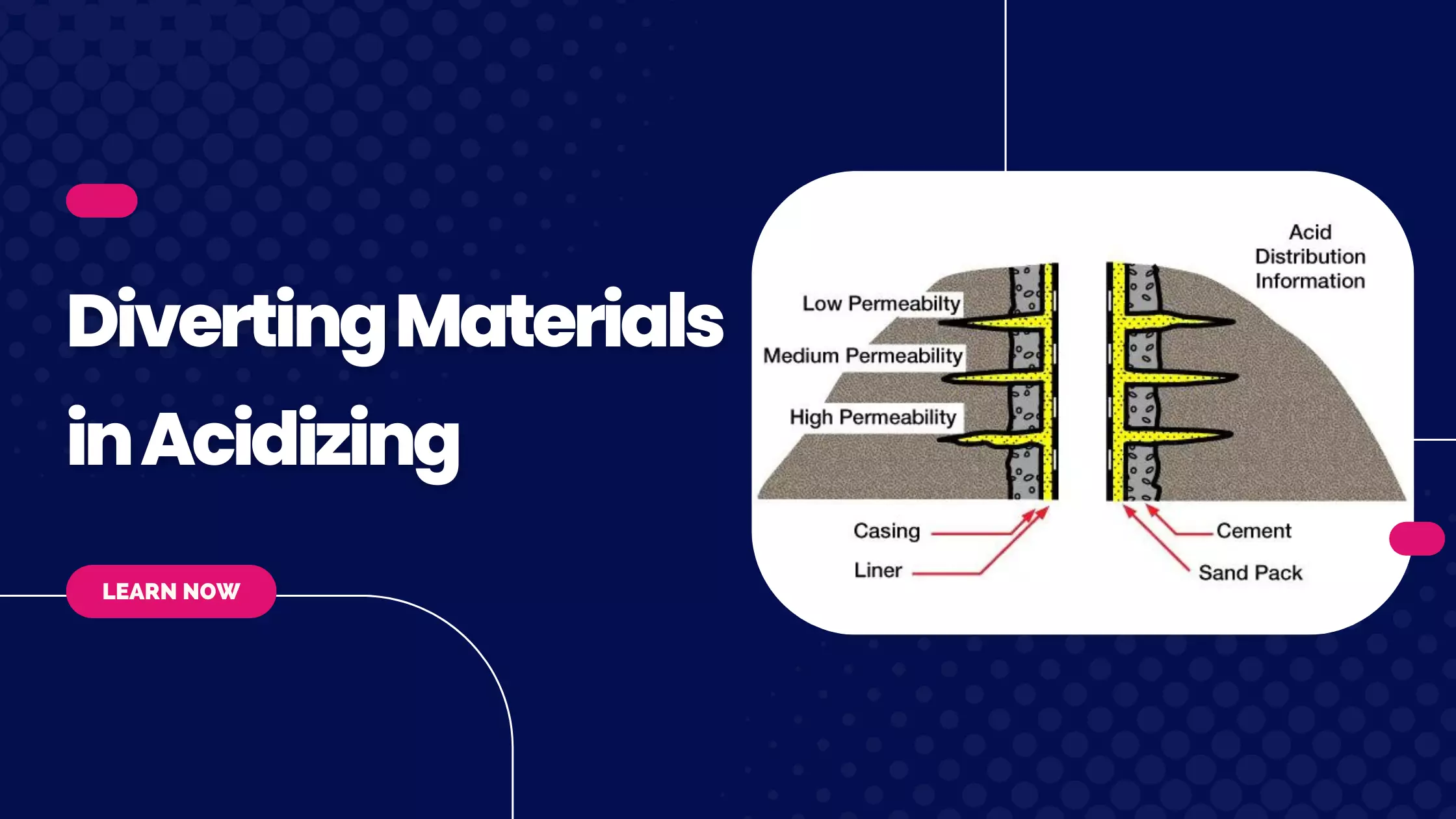
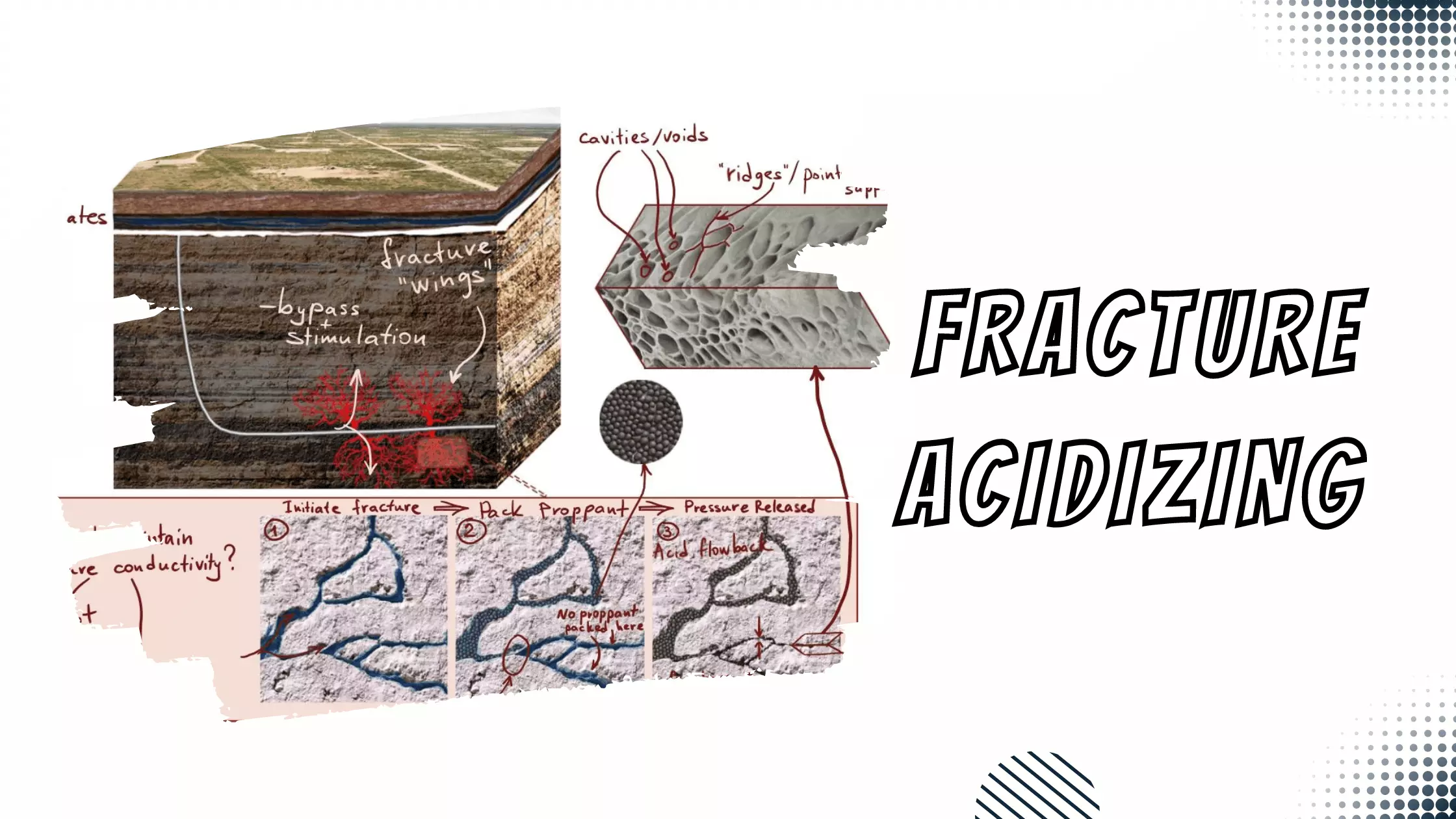
Iron Sequestering Agents
Iron occurs naturally in formation water, and in formation minerals such as siderite, hematite and pyrite. A primary source of iron, of course, is donwhole tubular goods. In solution, iron can exist in two forms: ferrous iron (Fe2+) and ferric iron (Fe3+). Fe3+ is or primary concern in acidizing, because it precipitates as gelatinous iron hydroxide (Fe(OH) 3) at a pH of 2.5 to 3.5. This precipitation can lead top formation damage.
When Fe3+is present in the near-wellbore region, an iron sequestrant, or chelating agent, may be added to the treating solution. Table 1 (below) provides information about various sequestering agents, of which EDTA is the most commonly used.
Because these agents can themselves damage the formation, they should be used only when pr-job evaluation and testing indicate a tendency for iron hydroxide precipitation during acidizing.
Other methods for keeping iron in solution include pH control and the use of oxygen scavengers. pH control is achieved by using a weak acid, such as acetic, which reacts much more slowly than HCl on limestone and other acid-soluble materials. Thus, some of the acid remains in solution, keeping the pH low and inhibiting iron Hydroxide formation.
| Compound | Maximum Solubility in 1000 gal 15% | Amount of Fe+2 Chelated – ppn |
|---|---|---|
| EDTA (Ethylene Diamine Tetraacetic Acid) | 67 | 1,450 |
| Sodium EDTA | ~250 | ~5,000 |
| NTA (Nitrilo Triacetic Acid) | 420 | 13,790 |
| Citric Acid | 1,796* | 57,292 |
*The solubility of citric acid is considerably higher than 1,796 lb. Theoretically, ferric iron in 15% HCl spent on millscale can be chelated with 1,796 lb of citric acid. However, if the acid was only partially spent on magnetite and the remainder spent on a carbonate rock, calcium citrate could precipitate.
Oxygen Scavengers: An oxygen scavenger can be used to economically control concentrations of iron up to approximately 5,000 mpl. It functions by two different mechanisms. First, it removes free oxygen from the fluid, and then helps prevent the oxidization of ferrous iron (F3+2)to ferric iron (Fe+3). Then, it acts as a reducing agent and reduces any dissolved ferric iron present to ferrous iron. This helps maintain iron in solution and helps prevent the precipitation of ferric iron. This helps maintain iron in solution and helps prevent the precipitation of ferric iron in solution. The amount of iron that can be reduced with an oxygen scavenger depends upon the quantity of chemical added, but it can be used up if the fluid is aerated before it is pumped.
A comparison of the amount of ferric iron that can be retained by the various methods and additives is illustrated in Figure 1 (iron cotnrol acid systems).
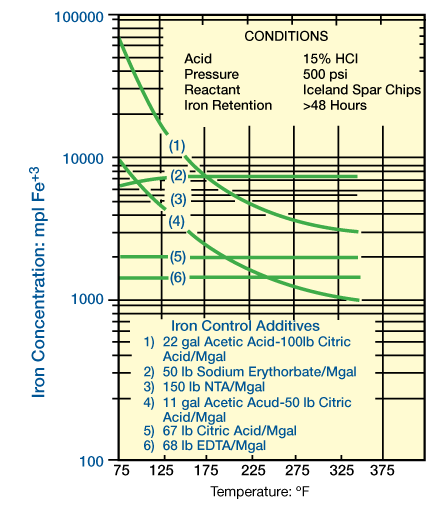
FIGURE 1
The safest way to help prevent damage to the reservoir from precipitated iron hydroxide is to clean or “pickle” the pipe with acid before acidizing the formation. This acid should contain large quantities of iron-control additives and should be circulated out of the well, not pumped into the formation. In conduction with this treatment, xylene or hydrocarbon phase should be incorporated or used as a preflush to remove pipe dope and varnish that could plug perforations. ( In most instances, pipe dope is not soluble in xylene or normal hydrocarbons.)
 Petro Shine The Place for Oil and Gas Professionals.
Petro Shine The Place for Oil and Gas Professionals.