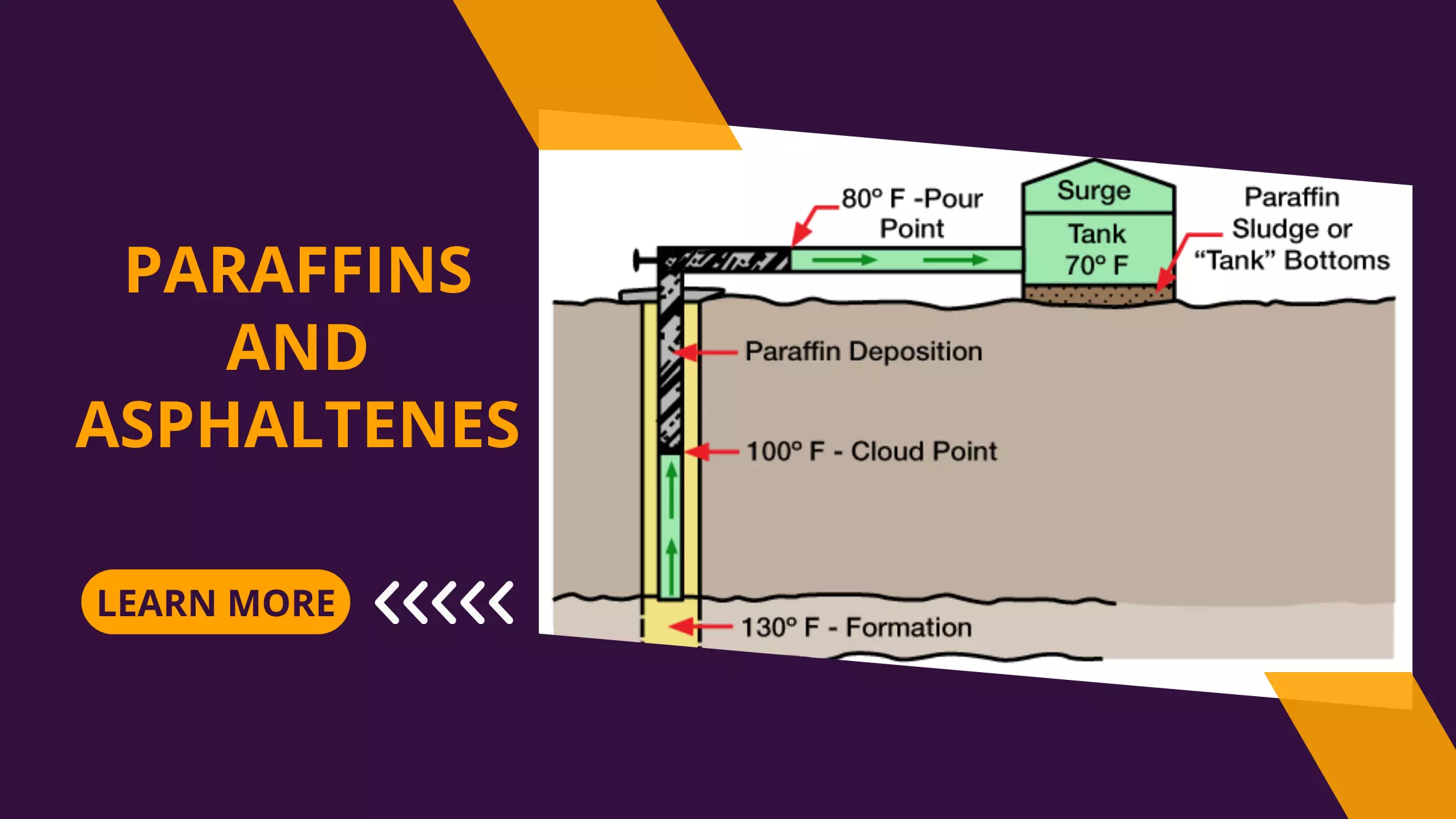
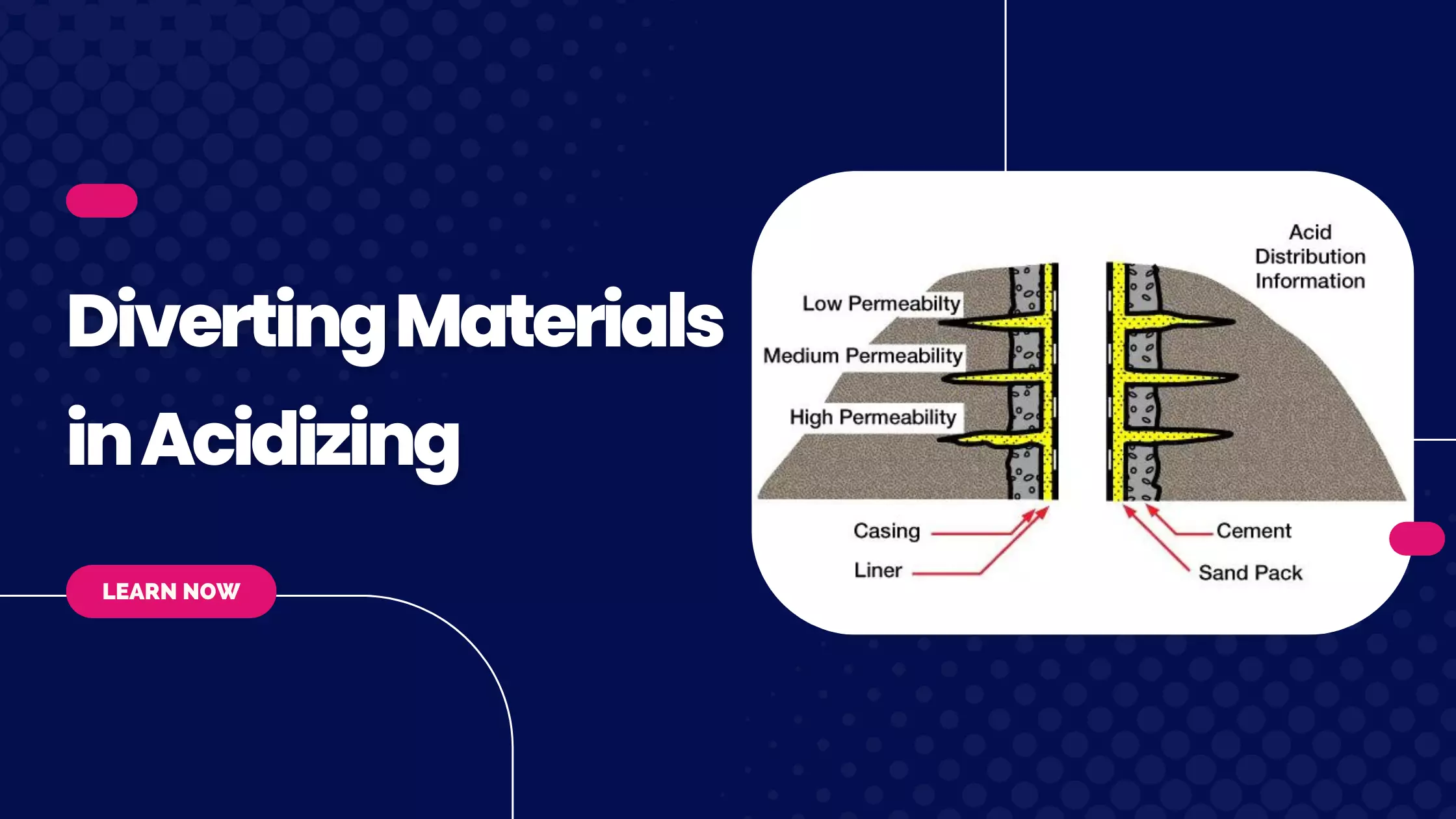
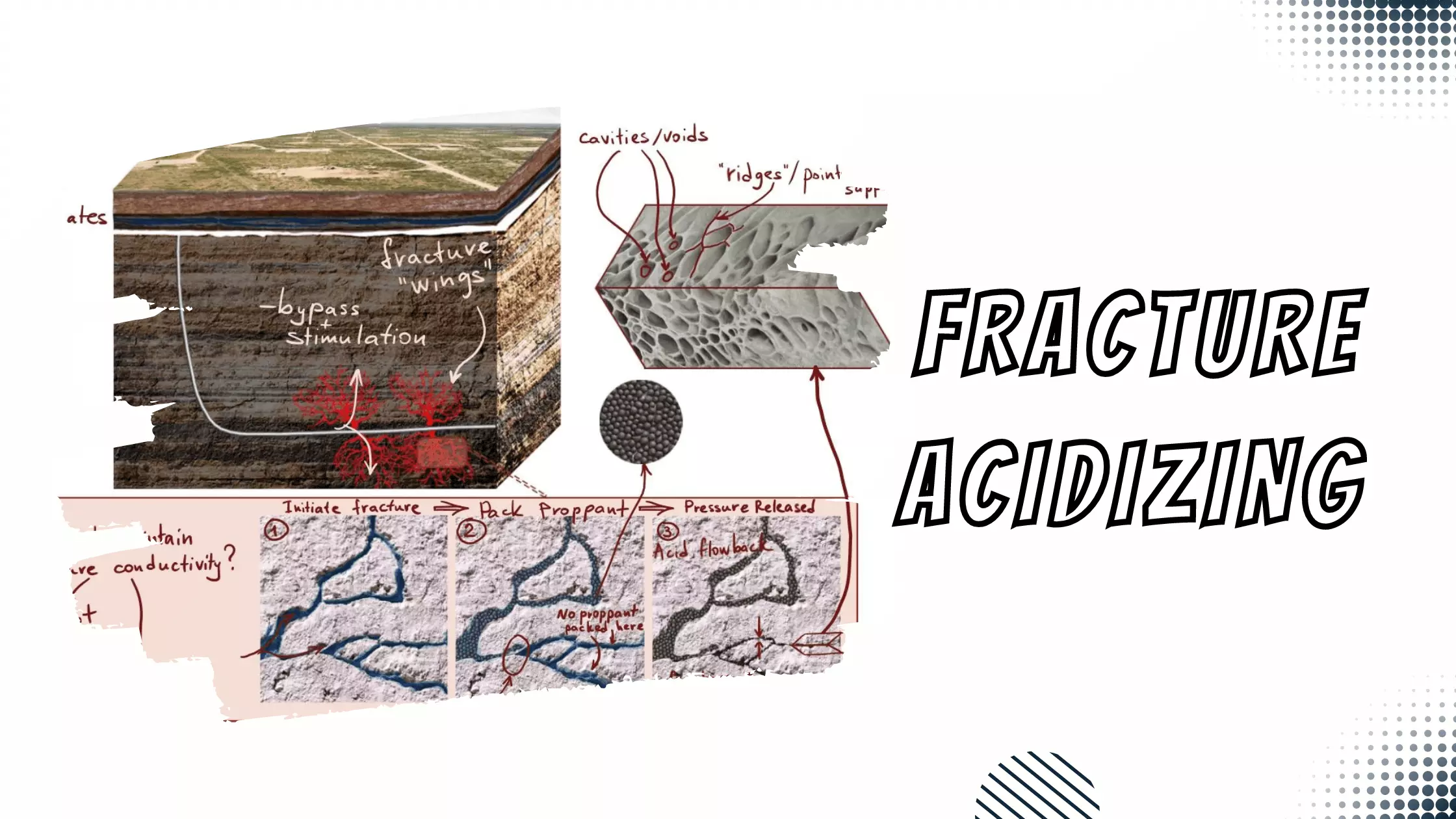
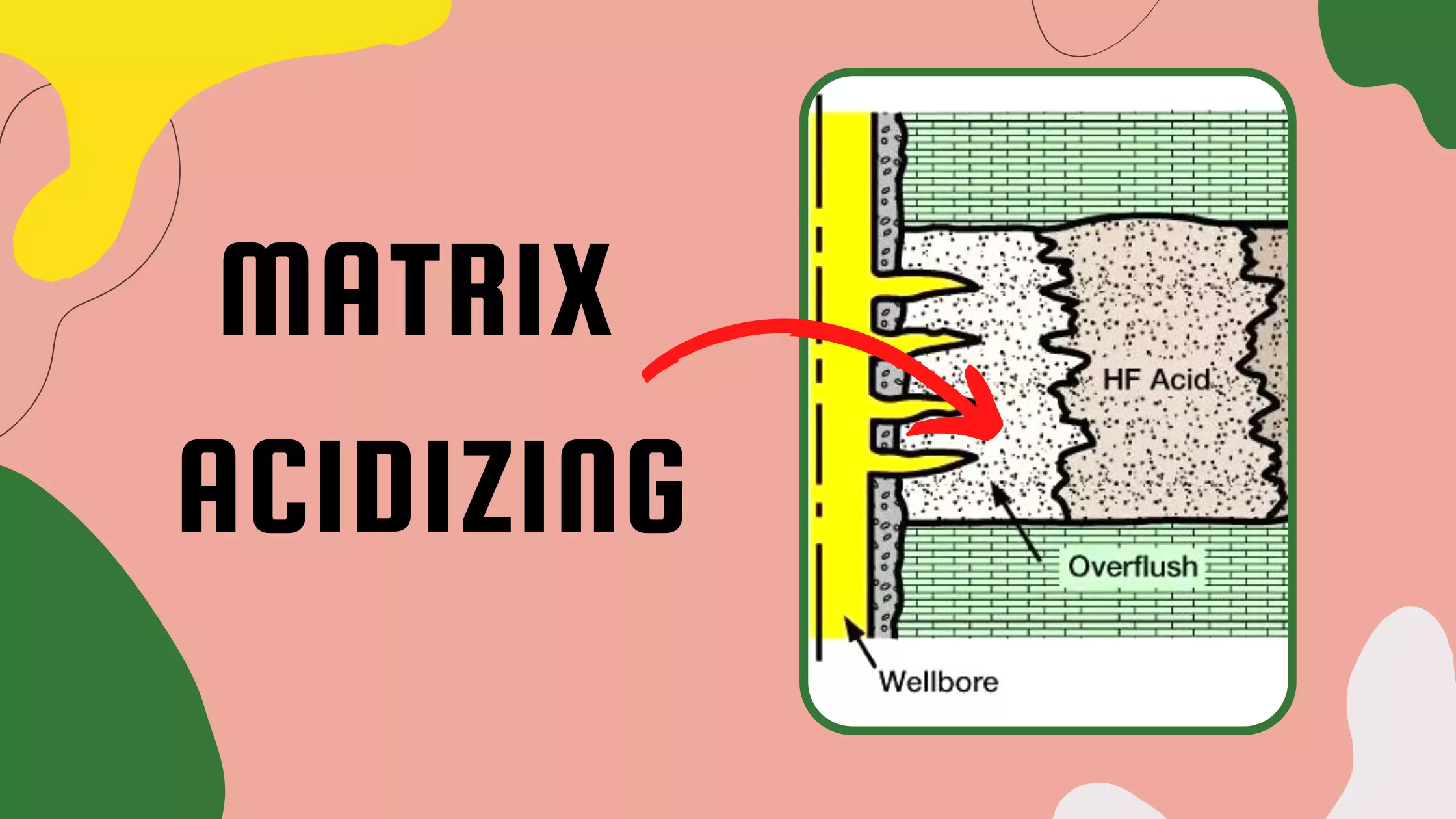
Scale Removal
Acid-soluble scales include the following:
- calcium carbonate
- iron carbonate
- iron sulfide
- iron oxides
- calcium hydroxide
These scales can be removed by one or more of the following solutions:
- 15% HCl with no surfactants HCl, containing only corrosion inhibitor to lower the corrosion rate, is a basic acid (without surfactants). It is not normally used for scale removal.
- 15% HCl with 0.15% penetrating agent This acid solution contains 1.5 gal of a penetrating agent per 1000 gal acid. Penetrating agents lower the surface tension of the acid solution, increasing the ability of acid to wet and spread. This solution is very effective on scales containing small amounts of iron. It can be used for all acid-soluble scale when treating surface, gathering lines, or any other system where the solution does not enter the formation.
- 15% HCl with sequestering agents This solution is used to prevent the precipitation of iron hydroxide. It is effective in handling scale that contains iron (Fe+2 and Fe+3) compounds. The HCl dissolves the iron scales. Sequestering agents (see Section 4.3) prevent iron precipitation by forming a complex with the iron, thus keeping it in solution and preventing secondary precipitation. With most iron scales, a stronger acid strength may also be considered.
- 10% acetic acid The greatest asset of this acid in scale removal is that it does not damage “chrome,” or alloy steel, found in downhole pumps. Calcium carbonate scales readily react with this acid, as do most other scales.
- 15% HCl -aroma tic dispersion This acid dispersion contains an aromatic solvent, acid, and an emulsifying surfactant.
Acid-insoluble scales include
- calcium sulfates
- barium sulfate
- strontium sulfate
- barium strontium sulfate
Acid-insoluble calcium sulfate can be removed by chemical methods; barium sulfate and strontium sulfate cannot.
There are two removal techniques for calcium sulfate (gypsum); one employs carbonate and hydroxide converter solutions, the other organic acid salts.
Carbonate and hydroxide converter solutions convert gypsum (CaSO4· 2H2O) to calcium carbonate, which is acid soluble. However, these converting properties are severely retarded when dense laminated gypsum scale is encountered. Because of a lack of penetration, repeated application may be necessary. In some cases, hydroxides form a sludge which can be pumped from the well; in others, acid must be used to remove the reaction precipitant. Although hydroxides have proved fairly effective in some wells, in others they tend to decrease in effectiveness with re-treatment. This is a result of a buildup of calcium hydroxides sludge within the interstices of the gypsum and on the surface, limiting solvent penetration.
With carbonate and hydroxide converter solutions, an acid stage is required to remove the reaction precipitate. These jobs are usually conducted in four stages, requiring a considerable amount of time.
Organic acid salts react with gypsum to form a reaction precipitate which tends not to adhere to the gypsum but to form a water-dispersible sludge. The reaction precipitant has a tendency to slough away from the gypsum surface, thereby increasing solvent penetration. Also, since the reaction product does not adhere tightly and is readily dispersible in water, an acid stage usually is not needed to remove the sludge. However, should it become necessary to remove the sludge, it is soluble to the extent of 1.2 lb/gal of 15% HCl.
When barium sulfate or strontium sulfate deposition has been diagnosed, the most practical solution is to eliminate the cause. For instance, barium sulfate is most often formed by the mixing of native formation water with injection or produced waters from another interval — one of which is high in barium while the other is high in sulfates – and isolating these waters can prevent the problem. The presence of barium sulfate usually indicates either a lack of zone isolation or faulty casing.
The removal of barium sulfate or strontium sulfate is an extremely difficult problem. Available chemicals that remove small amounts of these sulfates are usually inefficient and expensive. These deposits are best removed mechanically, by jetting or running a bit and scraper.
 Petro Shine The Place for Oil and Gas Professionals.
Petro Shine The Place for Oil and Gas Professionals.